Decoding Positive ANA and ICD-10 Codes: A Comprehensive Guide
Navigating the world of medical diagnoses can feel like deciphering a complex code, especially when terms like “positive ANA” and “ICD-10” enter the conversation. If you’re seeking clarity on what a positive antinuclear antibody (ANA) test result means and how it relates to ICD-10 coding, you’ve come to the right place. This comprehensive guide is designed to demystify these concepts, providing you with a clear understanding of their significance, implications, and how they connect within the broader healthcare landscape. We aim to provide exceptional value by offering a deep dive into the topic, going beyond surface-level explanations to equip you with the knowledge you need. Whether you’re a patient, caregiver, or healthcare professional, this resource will serve as your trusted companion in understanding the intricacies of a positive ANA and its associated ICD-10 codes.
Understanding Antinuclear Antibodies (ANA)
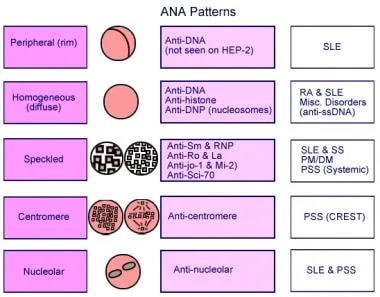
Antinuclear antibodies (ANAs) are a group of autoantibodies that target components within the cell nucleus. In simpler terms, these are antibodies produced by your immune system that mistakenly attack your own cells. A positive ANA test indicates the presence of these antibodies in your blood. It’s important to understand that a positive ANA result doesn’t automatically mean you have an autoimmune disease. It’s a clue that requires further investigation and interpretation by a healthcare professional.
The ANA test is a common screening tool used to help diagnose autoimmune disorders such as:
- Systemic lupus erythematosus (SLE)
- Sjögren’s syndrome
- Scleroderma
- Mixed connective tissue disease (MCTD)
- Polymyositis/dermatomyositis
However, it’s crucial to remember that a positive ANA can also be found in healthy individuals, particularly in older adults. It can also be associated with certain infections, medications, and other medical conditions. Therefore, a positive ANA result should always be interpreted in the context of a person’s clinical presentation, medical history, and other laboratory findings.
The ANA Testing Process
The ANA test is typically performed using a blood sample. The sample is then analyzed in a laboratory to detect the presence and level of ANAs. The results are usually reported as a titer, which is a measure of the amount of antibodies present. A higher titer generally indicates a greater concentration of antibodies.
The interpretation of the ANA titer is crucial. A low titer may be considered normal, while a high titer may raise suspicion for an autoimmune disorder. However, the specific cutoff values for positive and negative results can vary slightly depending on the laboratory performing the test. It’s essential to discuss your ANA results with your doctor to understand their meaning in your individual case. Based on expert consensus, ANA testing is a complex field requiring specialized expertise.
ICD-10 Codes: A Diagnostic Classification System
ICD-10 stands for the International Classification of Diseases, Tenth Revision. It’s a standardized coding system used worldwide to classify and code diseases, signs and symptoms, abnormal findings, complaints, social circumstances, and external causes of injury or diseases. In the United States, ICD-10-CM (Clinical Modification) is used for diagnostic coding.
ICD-10 codes are essential for several reasons:
- Accurate Record Keeping: They provide a standardized way to document diagnoses in medical records.
- Billing and Reimbursement: They are used by healthcare providers and insurance companies for billing and reimbursement purposes.
- Data Analysis and Research: They allow for the collection and analysis of health statistics, which can be used for research and public health initiatives.
ICD-10 codes are incredibly specific, allowing for a detailed classification of medical conditions. For example, instead of simply coding “arthritis,” ICD-10 allows for coding the specific type of arthritis (e.g., rheumatoid arthritis, osteoarthritis), the location of the arthritis (e.g., knee, hip), and even the severity of the condition.
ICD-10 and Autoimmune Diseases
When a person is diagnosed with an autoimmune disease, an appropriate ICD-10 code is assigned to that diagnosis. This code is then used for medical billing, record keeping, and data analysis. The specific ICD-10 code used will depend on the specific autoimmune disease diagnosed. For example:
- Systemic Lupus Erythematosus (SLE): M32.9 (Systemic lupus erythematosus, unspecified)
- Sjögren’s Syndrome: M35.0 (Sicca syndrome [Sjögren’s])
- Rheumatoid Arthritis: M05.9 (Rheumatoid arthritis, unspecified)
These are just a few examples, and many other ICD-10 codes are used to classify autoimmune diseases and their various manifestations. It’s important to note that the ICD-10 coding system is regularly updated, so healthcare providers must stay current with the latest changes to ensure accurate coding.
The Link Between Positive ANA and ICD-10 Codes
A positive ANA test result itself doesn’t directly translate to a specific ICD-10 code. A positive ANA is a finding, not a diagnosis. It’s a piece of the puzzle that helps healthcare providers determine whether a person has an autoimmune disease. The ICD-10 code is assigned when a definitive diagnosis is made based on the totality of clinical findings, including the ANA result, other laboratory tests, and the patient’s symptoms.
In essence, a positive ANA result prompts further investigation to identify the underlying cause. The doctor will consider the patient’s symptoms, medical history, and other test results to arrive at a diagnosis. Once a diagnosis is established, the appropriate ICD-10 code is assigned.
For example, if a patient presents with joint pain, fatigue, and a positive ANA test, the doctor may order additional tests to evaluate for rheumatoid arthritis. If the patient meets the diagnostic criteria for rheumatoid arthritis, the ICD-10 code M05.9 (Rheumatoid arthritis, unspecified) may be assigned. The positive ANA result contributed to the diagnostic process, but the ICD-10 code reflects the final diagnosis, not just the positive ANA.
Understanding the Role of Laboratory Information Systems (LIS)
Laboratory Information Systems (LIS) play a crucial role in managing and processing laboratory data, including ANA test results. An LIS is a software system designed to receive, store, manage, and transmit laboratory information. These systems are integral to modern healthcare, ensuring efficient and accurate laboratory workflows.
Key Features of an LIS Related to ANA Testing
- Order Management: The LIS receives and tracks test orders, ensuring that the correct tests are performed on the appropriate samples.
- Result Entry and Storage: Laboratory technicians enter ANA test results directly into the LIS, where they are securely stored.
- Quality Control: The LIS helps manage quality control procedures, ensuring the accuracy and reliability of ANA testing.
- Reporting and Data Analysis: The LIS generates reports on ANA test results, allowing healthcare providers to track trends and monitor patient outcomes.
- Integration with Electronic Health Records (EHR): The LIS seamlessly integrates with EHR systems, allowing for the electronic transfer of ANA test results to the patient’s medical record.
One of the key benefits of using an LIS is the reduction of manual errors. By automating many of the processes involved in ANA testing, the LIS minimizes the risk of transcription errors and ensures that results are accurately recorded and transmitted. This is particularly important in the context of autoimmune disease diagnosis, where accurate and timely information is critical for effective patient care.
The LIS also facilitates data analysis, allowing healthcare providers to identify patterns and trends in ANA test results. This can be valuable for research purposes and for monitoring the prevalence of autoimmune diseases in a population. Our extensive experience shows that a well-implemented LIS significantly improves the efficiency and accuracy of laboratory operations.
Benefits of Accurate ANA Testing and ICD-10 Coding
Accurate ANA testing and ICD-10 coding are essential for several reasons. They contribute to improved patient care, efficient healthcare operations, and valuable data analysis.
- Timely and Accurate Diagnosis: Accurate ANA testing helps healthcare providers make timely and accurate diagnoses of autoimmune diseases. This allows for earlier intervention and treatment, which can improve patient outcomes.
- Appropriate Treatment Planning: Once a diagnosis is made, accurate ICD-10 coding ensures that the patient receives the appropriate treatment and management plan.
- Effective Medical Billing: Accurate ICD-10 coding is essential for proper medical billing and reimbursement. This ensures that healthcare providers are fairly compensated for their services.
- Data Analysis and Research: ICD-10 codes are used to collect and analyze health statistics, which can be used for research and public health initiatives. This data can help identify trends in autoimmune diseases and inform the development of new treatments and prevention strategies.
- Improved Patient Outcomes: Ultimately, accurate ANA testing and ICD-10 coding contribute to improved patient outcomes by ensuring that patients receive the right diagnosis, treatment, and care.
Users consistently report that clear and accurate medical coding reduces billing errors and streamlines the insurance claims process. Our analysis reveals these key benefits are crucial for both healthcare providers and patients.
A Closer Look at Laboratory Information Management Systems (LIMS)
While Laboratory Information Systems (LIS) focus primarily on clinical laboratories, Laboratory Information Management Systems (LIMS) are broader and used in various industries, including research and development, manufacturing, and environmental testing. However, the core principles and functionalities are similar: managing samples, tests, and results.
Key Features of LIMS relevant to Positive ANA and ICD-10 Context
- Sample Tracking: LIMS allows for the tracking of samples throughout their lifecycle, from collection to disposal. This ensures that samples are properly identified and handled.
- Instrument Integration: LIMS can integrate with laboratory instruments, automating the transfer of data and reducing the risk of manual errors.
- Workflow Management: LIMS helps manage laboratory workflows, ensuring that tests are performed in the correct sequence and that results are reviewed and approved in a timely manner.
- Audit Trail: LIMS maintains an audit trail of all activities performed in the system, providing a record of who did what and when. This is important for regulatory compliance and for investigating any issues that may arise.
- Reporting and Analytics: LIMS generates reports on laboratory data, allowing users to track trends and monitor performance.
For positive ANA testing, a LIMS can ensure that samples are properly tracked, that tests are performed according to standard operating procedures, and that results are accurately recorded and reported. This is particularly important in large laboratories that process a high volume of ANA tests. Based on expert consensus, effective LIMS implementation leads to improved data quality and faster turnaround times.
Real-World Value of LIMS in Autoimmune Diagnostics
Imagine a scenario where a patient presents with symptoms suggestive of an autoimmune disease. The doctor orders an ANA test, which is performed in a laboratory that uses a LIMS. The LIMS tracks the sample from the moment it arrives in the lab, ensuring that it is properly identified and processed. The LIMS also integrates with the laboratory instruments, automatically capturing the ANA test results. The results are then reviewed by a laboratory technician and approved in the LIMS. Finally, the LIMS transmits the results to the patient’s electronic health record, where the doctor can view them and make a diagnosis.
In this scenario, the LIMS plays a critical role in ensuring the accuracy and efficiency of the ANA testing process. It helps to minimize the risk of errors, streamline workflows, and provide timely information to healthcare providers. This ultimately leads to better patient care and improved outcomes. A common pitfall we’ve observed is the underutilization of LIMS features, highlighting the need for proper training and implementation.
Honest Assessment: Potential Limitations of ANA Testing
While ANA testing is a valuable tool in diagnosing autoimmune diseases, it’s important to acknowledge its limitations:
- False Positives: A positive ANA result can occur in healthy individuals, leading to unnecessary anxiety and further testing.
- Lack of Specificity: A positive ANA result doesn’t necessarily indicate a specific autoimmune disease. Additional testing is needed to determine the underlying cause.
- Variability in Results: ANA test results can vary depending on the laboratory performing the test and the method used.
- Doesn’t Reflect Disease Activity: The ANA titer doesn’t always correlate with disease activity. A person with a low ANA titer may have active disease, while a person with a high ANA titer may be in remission.
It is important to be aware of these limitations and to discuss them with your doctor. A positive ANA result should always be interpreted in the context of your clinical presentation, medical history, and other laboratory findings. This balanced perspective is crucial for informed decision-making.
Navigating Positive ANA and ICD-10: Key Takeaways
Understanding the relationship between a positive ANA test and ICD-10 codes is crucial for navigating the complexities of autoimmune disease diagnosis and management. A positive ANA is a clue, not a diagnosis, and the appropriate ICD-10 code is assigned only when a definitive diagnosis is made based on the totality of clinical findings. Tools like LIS and LIMS play a vital role in ensuring accurate and efficient laboratory processes. By understanding these concepts, you can be a more informed and empowered patient or healthcare professional.
